MicroCurrent Stimulation 100ile Rent-to-Purchase Option (Total Price $1,350)
Microcurrent Stimulation 100ile Rent-To-Purchase Option - cost is $100 per month. First payment is $475 plus $12 shipping. This includes a $375 security deposit, which is refunded if any time during the rental the unit is returned in good condition. Then the charge is $100 per month for the next 9 months until the unit is fully paid off. Total cost of the unit is $1350.
100% of the rental and security deposit is applied against the purchase price. The customer has the option to return the unit, and the customer would only be charged $100 per month for the months used, and the security would be returned.
MicroCurrent Stimulation (MCS) is an enhanced adaptation of an FDA-approved therapy used by anesthesiologists, orthopedic surgeons, plastic surgeons, and rehabilitative specialists to promote the healing of wounds and transplanted tissues and treat pain. Customer Experiences
WHAT IS UNIQUE ABOUT THIS UNIT
This is the only unit based on all research studies done to date. It has a unique Regulator and Processor that uniquely adjusts the microcurrent based on each individual's retinal tissue acceptance of the microcurrent (1,000 times per second). The higher frequencies in the beginning of each 5-minute session helps relax the retinal tissue, then the lower frequencies stimulate the retina and ATP (energy) production within the retinal cells. The treatments also attract adult stem cells to help stimulate possible cell regeneration.
HOW MCS WORKS
The theory is that MCS helps:
- re-stimulate and energize dormant retinal cells (cells are like batteries -- when they run low in energy, they become sluggish and dormant),
- boost the cells' ability to rid themselves of waste products that interfere with the flow of energy, nutrients and communication,
- increase blood supply to the area stimulated. By increasing blood flow to the area, cells, and tissues still living can be nourished and refreshed.
Although the exact mechanism of action of MCS has not yet been established scientifically, research suggests that this microcurrent electrical stimulation device approximates the level of electrical activity present in a healthy eye. This is believed to stimulate retinal activity, energize dormant cells, and improve microvascular circulation, nerve conduction, and velocity.
Microcurrent stimulation increases ATP (energy) synthesis in the retinal cells needed for membrane viability and waste management (a major concern for those with dry macular degeneration, in which excess waste not reabsorbed and eliminated, resulting in the accumulation of waste called "drusen").
The treatment of patients with Macular Degeneration and Retinitis Pigmentosa entails the periodic administration of very precise amounts of tightly controlled electrical current through electrodes applied to the skin at specific areas around the eye. The electrical current is used to stimulate the retina and the diseased macula in order to help protect sight. The procedure is safe, non-invasive, and painless. No side effects or adverse reactions have been observed.
WHY WE OFFER THIS PARTICULAR UNIT:
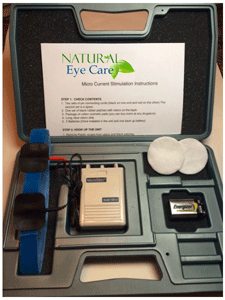
This is the home unit version of the Microcurrent Stimulation (MCS 100 ile). The frequency settings were used in the studies described below. It is preset automatically to run through 4 frequencies in a 5-minute cycle before turning off. The four frequencies are: 292Hz for 30 seconds, 30Hz for 30 seconds, 9.1Hz for 2 minutes, then finally .3Hz for 2 minutes. Voltage is up to 22 and amps range 700 microAmps.
There are other microcurrent stimulation units available, but they have to be specifically calibrated both in frequency and timing to ensure proper gentle wave currents for your eyes. Most of these units are actually TENS units designed more for wound healing and bone repair.
IS MICROCURRENT STIMULATION SAFE:
No side effects or adverse outcomes related to this treatment have been seen so far. No increase in the conversion to the wet form of ARMD has been seen in those who have used microcurrent stimulation properly.
A consensus statement by the National Institutes of Health reports that "One of the advantages...is that the incidence of adverse effects is substantially lower than that of many drugs or other accepted medical procedures used for the same conditions."
RESEARCH
See research listed under the purchase option for this product.
FDA POSITION
MCS therapy is considered an "off-label" use of an approved medical device. Off-label usage of FDA-approved devices and drugs is commonly practiced by doctors without interference from the FDA. This allows doctors to practice in a manner they believe is most beneficial to their patients.
DIAGNOSIS:
When ordering, please let us know your specific eye diagnosis.
CLIENT EXPERIENCES:
"I just returned from my annual eye exam with my neuro-ophthalmologist and I'm thrilled to tell you that my eyesight has stabilized---there is no further progression of macular degeneration! I continue my regime daily, without fail, two microcurrent stimulation sessions, 3 pumps of Pure Focus, plus a plethora of anti-oxidants."--More from MCS Users.
CONTRAINDICATIONS:
MCS is contraindicated for those with pacemakers, or who are pregnant. If you have a neurological disorder such as epilepsy, please discuss it with your doctor first before using MCS.
DISCLAIMER:
The data presented here has not been reviewed by the FDA, nor has it been peer-reviewed. The microcurrent devices used are approved by the FDA for the treatment of pain, but they have not been approved for other uses. The use of a device for off-label use by a physician is legal. The use of microcurrent stimulation discussed here is only one part of a comprehensive program for supporting visual health, and should not replace any treatment prescribed by your physician.
Warranty
There is a full 1-year replacement warranty on any defective unit (based on normal wear and tear).
Client Comments
"My name is Max Mandolini, and I am a Vision Educator in Long Beach, California. My father Leandro, who lives in Italy, was diagnosed with a form of Wet AMD in his left eye. He is 83, in good overall health: his major problem was an operation for a heart condition in 1998. After that, he has been taking some blood thinning drugs that may have contributed to leakage in the macular area.
"Late in June 2006, he received an endovitreous injection of Avastin (a steroid-type drug). At that time his vision in OS was 1/10 (Italian measurement). Late in July, I visited him and he started taking some supplements (EPA DHA, Lutein, and an eye formula). Also, he started a daily treatment of MCS ile 1000, which I had purchased from NaturalEyeCare.
"In September, Leandro started complaining of deterioration of vision in his other eye (OD), and was given an injection of Avastin. At that point, he almost could not read and write, and even insecure when walking outside.
"He continued his regime of daily supplementation and MCS treatment. Early in November, he told that he was noticing an improvement in his vision. His latest medical exam (Nov 30) confirmed the improvement to an unpredictable extent: his vision had improved from 20/200 to 20/40.
"The doctor, dumbfounded, has scheduled an appointment for an OCT scan in January. My father, overjoyed, continues his regime."
-- Max Mandolini, NEI Educator - 1/7/07
| Free 1st Class Shipping | Free 1st Class Shipping |
|---|